- This topic is empty.
-
AuthorPosts
-
2025-10-17 at 11:20 am #10914
6-Aminonicotinaldehyde (CAS No.: 69879-22-7) is an important heterocyclic compound widely used as a versatile intermediate in organic synthesis. With the molecular formula C6H6N2O and a molecular weight of 122.12 g/mol, this compound combines two highly reactive functional groups—an amino group and an aldehyde group—within a pyridine ring system. Its reactivity allows for a broad spectrum of transformations, making it essential in pharmaceuticals, fine chemicals, and advanced materials. In this blog post, as high purity industrial fine chemicals supplier, SACH will share the properties of 6-Aminonicotinaldehyde for sale, its synthesis methods, applications, etc.
Physical Properties of 6-Aminonicotinaldehyde
* Standard Purity: 98%
* Appearance & Packaging: Supplied in 25 kg drums for industrial use
* Melting Point: 161 ℃
* Boiling Point: 309.0 ± 27.0 ℃ (Predicted)
* Density: 1.264 ± 0.06 g/cm³ (20 ℃, 760 Torr)
* Acidity Coefficient (pKa): 4.78 ± 0.13 (Predicted)
* Storage Conditions: Stable under inert gas (nitrogen or argon) at 2–8 ℃
These features establish 6-Aminonicotinaldehyde as a stable yet reactive chemical for both laboratory and industrial applications.
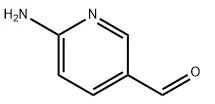
Chemical Properties of 6-Aminonicotinaldehyde
The dual functionality of 6-Aminonicotinaldehyde underpins its wide reactivity profile.
* Amino Functionality: Readily undergoes condensation with aldehydes and ketones to form Schiff bases, which are key intermediates in bioactive compounds.
* Aldehyde Functionality: Capable of participating in acetal formation with alcohols, as well as further oxidation to carboxylic acids.
* Stability Concerns: While stable under controlled conditions, it may oxidize in air and is sensitive to hydrolysis under acidic or alkaline environments.
This balance of stability and reactivity makes it attractive for controlled synthesis strategies.
Synthesis Methods of 6-Aminonicotinaldehyde
Method 1: From 3-Cyano-5-Ethoxycarbonylpyridine
1. Hydrolysis: 3-cyano-5-ethoxycarbonylpyridine undergoes hydrolysis in sodium hydroxide solution to produce the corresponding acid.
2. Reduction: The carboxyl group is reduced to an aldehyde.
3. Amination: Introduction of the amino group provides the target compound, 2-amino-5-aldehyde pyridine.
This route highlights the efficiency of sequential hydrolysis, reduction, and amination reactions.
Method 2: From 2-Aminopyridine
1. Protection: The amino group is first protected to avoid side reactions.
2. Halogenation: A halogen atom is introduced at the 5-position of the pyridine ring.
3. Reduction: The halogen is then converted into an aldehyde group.
4. Deprotection: Finally, the amino protecting group is removed, yielding the desired 6-Aminonicotinaldehyde.
This method emphasizes selectivity by using protective groups to ensure clean product formation.
Applications of 6-Aminonicotinaldehyde
Organic Synthesis Applications
As a synthetic intermediate, 6-Aminonicotinaldehyde enables the preparation of diverse heterocycles. By modifying the pyridine ring with different substituents, researchers can design novel derivatives with specialized applications in dyes, pesticides, and fine chemicals.
Role in Drug Synthesis
In the pharmaceutical field, 6-Aminonicotinaldehyde plays a pivotal role in drug discovery and development:
* Antiviral Agents: Its aldehyde group serves as a reactive center for coupling reactions, enabling the construction of antiviral scaffolds.
* Antitumor Compounds: The amino group participates in condensation pathways critical for forming heteroaromatic drug candidates.
* Medicinal Chemistry Intermediate: As a building block, it supports the synthesis of complex molecules with enhanced bioactivity.
Analytical Chemistry Applications
In analytical chemistry, 6-Aminonicotinaldehyde functions as:
* Chromogenic Agent: Useful in metal ion detection through coordination reactions.
* Extractant: Facilitates separation of specific ions, aiding in qualitative and quantitative analysis.
Its ability to form colored complexes improves its value in analytical testing.
Materials Science Applications
Beyond pharmaceuticals, this compound also impacts materials science:
* Electroluminescent Materials: The conjugated pyridine structure contributes to efficient light emission.
* Liquid Crystals: Structural modifications of 6-Aminonicotinaldehyde derivatives provide liquid crystal materials with enhanced optical properties.
* Functional Polymers: Its reactive groups allow it to be embedded into polymer chains, offering tailored electronic and mechanical performance.
Conclusion
6-Aminonicotinaldehyde (CAS No.: 69879-22-7) is more than just a chemical compound—it is a cornerstone intermediate in modern synthetic chemistry. With dual functional groups, reliable synthesis pathways, and broad applications ranging from pharmaceuticals to advanced materials, it continues to attract attention from researchers and industries alike. Its strategic role in building complex molecules highlights its ongoing relevance in drug discovery, analytical chemistry, and functional material innovation.
-
AuthorPosts
- You must be logged in to reply to this topic.